This is to inform the General Public that the following products are approved for withdrawal, suspension and cancellation by NAFDAC.
They are therefore no longer permitted for manufacture, importation, exportation, distribution, advertisement, sale and use within Nigeria. Please note that:
1. The Certificate of Registration of a product is said to be withdrawn when the use of the Certificate of Registration of that product is discontinued upon request of the Market Authorization Holder.
2. The Certificate of Registration of a product may be suspended when the conditions upon which the NAFDAC Registration license was issued are no longer met and the Agency is to make a determination.
3. The Certificate of Registration of a product is said to be cancelled when the NAFDAC Certificate of Registration license of that product is revoked by NAFDAC.
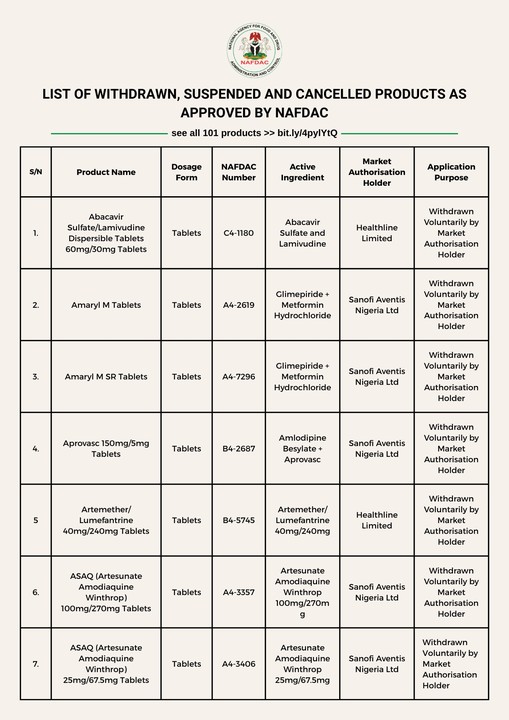
See Full List: https://nafdac.gov.ng/list-of-products-withdrawn-by-nafdac/

